This can also be used to determine the shelf life of a product prior to purchase. The pqri stability shelf life (ssl) working group was formed in late 2006 to investigate current and alternative statistical methods for estimating the shelf life of pharmaceutical products with stability data.

Kinetics And Drug Stability Ed
Enter all three dates to the left to see the.

Shelf life calculator for pharmaceutical products. For the products stored at room temperature, the assessment should be started from the occurrence of the significant change in product stored in accelerated conditions. Pharmaceutical industry, even after sitting on the shelf for a long time or when exposed to various. Any suitably conservative estimate of the true product shelf life, as supported by statistical calculations, is called the supported shelf life.
Stability the usp defines the stability of a pharmaceutical product as “ extent to which a product retains , with in specified limits, and throught out. “a measure of how pharmaceutical product maintains its quality attribute over a time”. Pacific coast composites’ shelf life calculator is provided in order to help our customers determine the remaining shelf life of their product.
Shelf life is a product of physical, microbiological, and chemical processes, triggered by any one of a multitude of contributing factors. In order to calculate expiry date, you should look at production date on your wrapping and write it into relevant field. In general, this estimate of the true product shelf life is called the estimated shelf life or the estimated product shelf life.
Expiry with date in days, in months or in years. The shelf life of food, pharmaceuticals, and other perishable items is defined with one of the factors, i.e., permeability or flux. After entering data you push.
Every packaging engineer | technologist should know the calculation of. Also it is necessary to find the mark: From these data, an estimate of the true product shelf life is obtained.
The product may be released into the market after accelerated stability studies but the shelf life should also be verified simultaneously using long time studies. (1) the need to make statistical approaches more Stability testing and shelf life estimation.
This can also be used to determine the shelf life of a product prior to purchase. It is intended to be a conservative. A pharmaceutical product may undergo change in appearance, consistency, content explanation/ reason increase in concentration.
Product name shelf life start date * today's date * expiration date *. This document assists with establishing the expiration period of a production bath of a medicinal product. 151 152 finished pharmaceutical product (fpp) 153 a product that has undergone all stages of production, including.
1995 february 1996 transmission to the cpmp june 1996 Minimum data of three batches are used to estimate the shelf life of pharmaceutical products. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood and plasma, as well as.
This guideline applies to human and veterinary medicines. Drug stability manish kumar sharma maip jaipur. The wall thickness and grade of the plastic depends on how much air, moisture, and organic vapor can diffuse through the packaging material.
This online service helps you to know how long your product is in good condition. Pacific coast composites’ shelf life calculator is provided in order to help our customers determine the remaining shelf life of their product. The interest in alternative methods was stimulated by several factors:
Between manufacture and usage of the product. Shelf life the label modification of any factor uniformity, clarity (solution), moisture contents, particle size and

Ask The Expert Utilizing Reusable Real-time Technology To Reduce Supply Chain Waste Controlant Cold Chain As A Service

Kinetics And Drug Stability Ed

Shelf Life Calculation Of Drugs
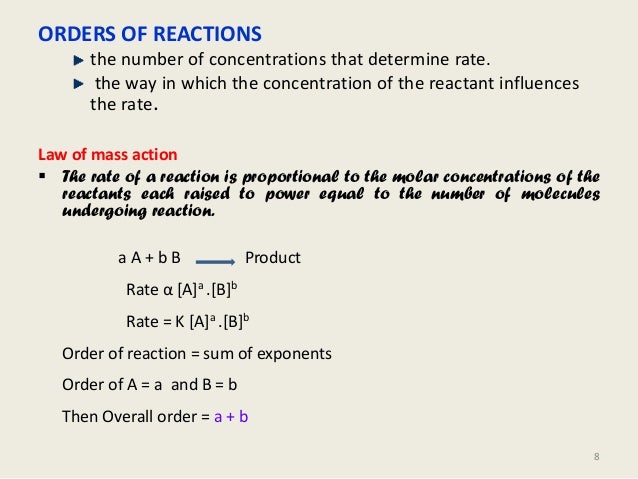
Kinetics And Drug Stability Ed

Shelf Life Calculation Of Drugs

Assessing Shelf Life Using Real-time And Accelerated Stability Tests

Kinetics And Drug Stability Ed

Kinetics And Drug Stability Ed

Calculation Of Expiry Date Shelf-life Of Medicine By Accelerated Stability Study Method In English – Youtube
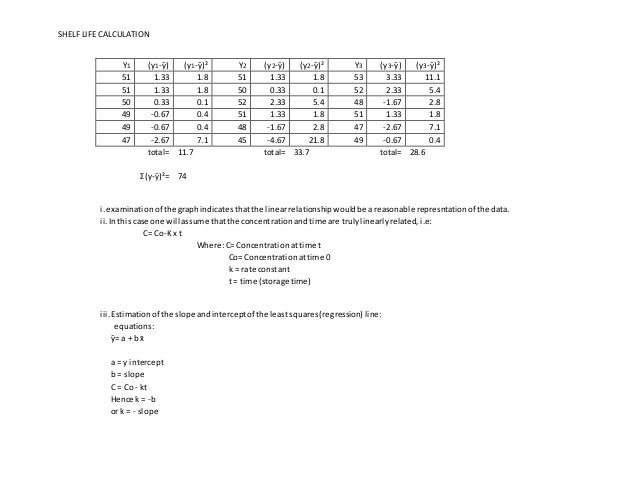
Shelf Life Calculation Of Drugs

Shelf Life Calculation Of Drugs

Shelf Life Calculation Of Drugs

Shelf Life Calculation Of Drugs
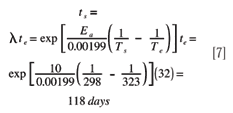
Assessing Shelf Life Using Real-time And Accelerated Stability Tests

Shelf Life Calculation Of Drugs

How To Determine The Exact Shelf Life Of Cosmetics From The Moment Of Production And After Opening The Package The Shelf Life Of Cosmetics And Decoding On The Batch Code To Know

Shelf Life Calculation Of Drugs

How To Determine The Exact Shelf Life Of Cosmetics From The Moment Of Production And After Opening The Package The Shelf Life Of Cosmetics And Decoding On The Batch Code To Know









